Polymer Oxidation
Polymer is processed or exposed to harsh environment due to heat, high energy radiation (light), mechanical shear, residual catalyst, etc., which causes the polymer to chemically react or degrade. These reactions or degradation represent changes in chemical structure and molecular weight of the product, resulting in deterioration of mechanical properties, and also the product’s appearance may be yellowing or gloss reduction.
Chain Reaction
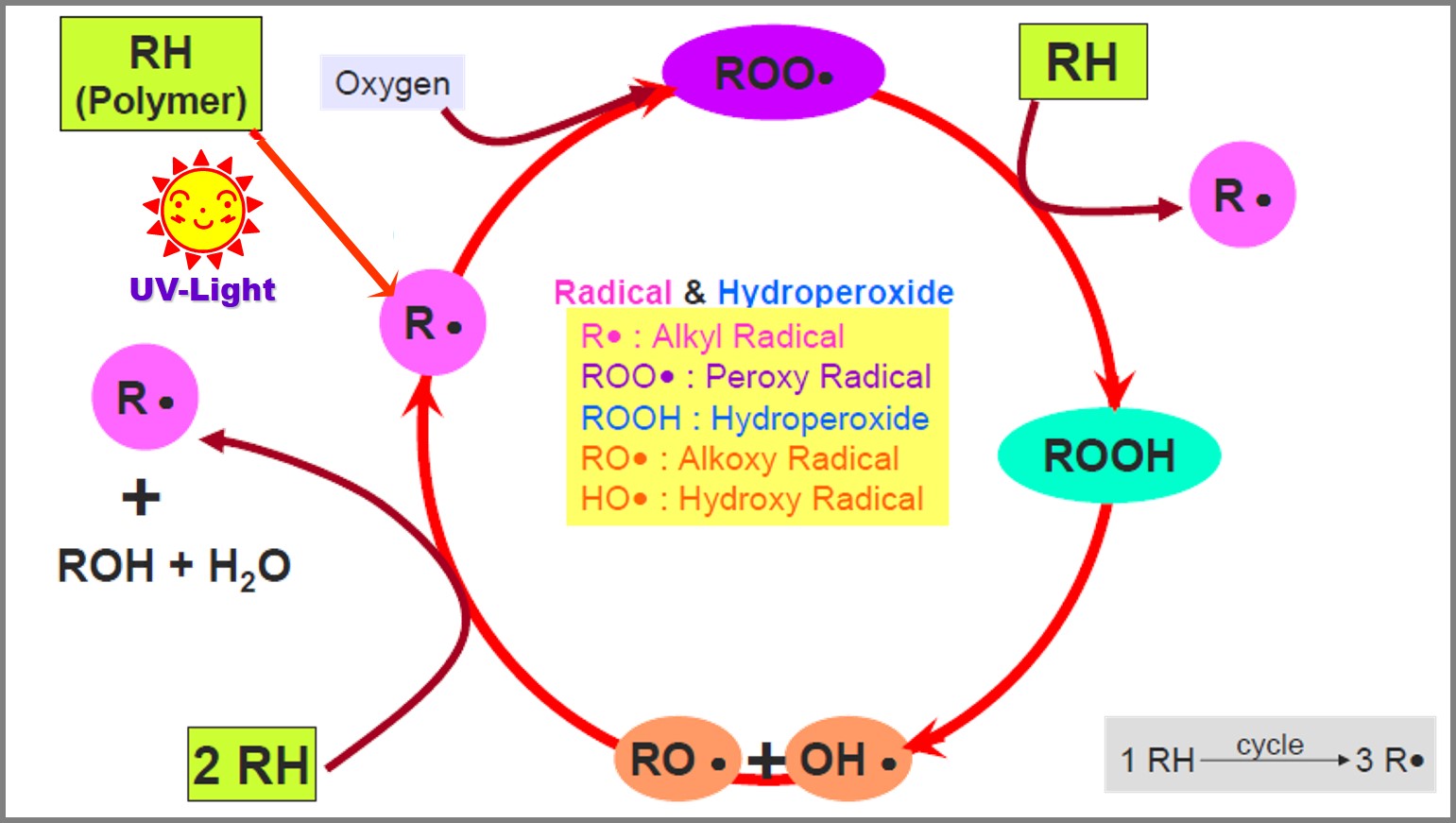
Free radical and hydroperoxide are highly active intermediates. Degradable polymers generate free radicals that react with free oxygen from environment to form more free radicals and peroxides. These chemicals will damage the polymer structure, degrading it, but also generate new free radicals, continuing a vicious circle. Antioxidants are additives that are primarily intended to prevent and protect polymer from degradation. In accordance with their reaction mechanism, antioxidants mainly divide into three types: Primary Antioxidant, Secondary Antioxidant and Carbon-centered Antioxidant, these different types of antioxidants are used in different stages of preservation in polymer oxidation process.
1. Carbon-centered Antioxidant
Also known as carbon-centered free radical scavenger, when polymer structure degrades into carbon-centered free radicals by heat, high energy radiation, mechanical shear, residual catalyst, and so on, the antioxidant captures the free radicals immediately. Therefore, carbon-centered Antioxidant is powerful and high efficient antioxidant.
2. Primary Antioxidant
Primary antioxidant, such as hindered phenols and amines, is to quickly coupling reaction (that is, to terminate the reaction) or to give a hydrogen atom to form a relatively inert compound to terminate the damage from peroxide radicals (ROO ˙), thus stopping the chain reaction. It is also known as " Chain-Terminated Antioxidants ".
3. Secondary antioxidants
Secondary antioxidants, such as phosphites and thioethers, are also known as "preventative antioxidants." These types of antioxidants are easily to react with hydroperoxide groups (ROOH) to form inert, non-free radical compounds that terminate the autooxidation-chain reaction. Usually, phosphites are used in melting process combining with hindered phenolic antioxidants to reach the best result.
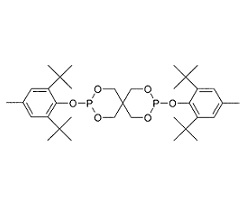
| CAS No. | 80693-00-1 |
| Appearance | White powder |
| Melting Point | 236 ~ 239°C |
| Loss on Drying | 0.3% max. |
It provides excellent low color and melt stability during polymer processing as well as stable hydrolytic stability and outstanding thermal stability. Eunox HP-36 protects polymers against thermal degradation during high temperature processing, and it suppresses discoloration of polymers caused by thermal oxidation during long time use, also it provides color stability under the environment of NOx gas.
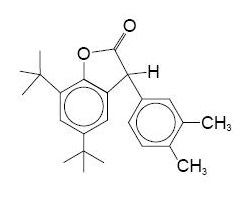
| CAS No | 181314-48-7 |
| Appearance | White powder |
| Melting Point | 130 |
| Loss on Drying | 0.5% max. |
Eunox AO-136 can be used in specialty polymers, e.g., polycarbonate, polyesters, blends and alloys of PC and PES, polyurethane, styrenics, elastomers and adhesives
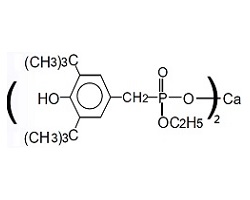
| CAS No | 65140-91-2 |
| Appearance | White to off-white powder |
| Melting Point | >270℃ |
| Loss on Drying | 0.5% max. |
Eunox AO-1425 is a multifunctional additive designed for the manufacture of polyester resins (bottle, fiber, film, industrial yarns, sheet, strapping … etc.), melt processing of polyesters (fiber spinning, extrusion, injection molding … etc.), synthesis of rosin esters and the stabilization of adhesives, tackifiers and rubber vulcanisates. Eunox AO-1425 is odorless, stable to light and has excellent color retention. It has good compatibility with most substrates and high resistance to extraction.